Problem
In the reaction,
$P + Q \to R + S$
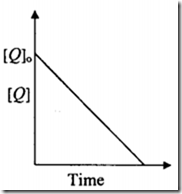
the time taken for 75% reaction of P is twice the time taken for 50% reaction of P. The concentration of Q varies with reaction time as shown in the figure. The overall order of the reaction is
(A) 2 (B) 3
(C) 0 (D) 1
Solution
Since, $\frac{{d[Q]}}{{dt}} = const.$ the order does not depend on [Q]. Clearly, the reaction is zero order w.r.t. Q.
For first order reaction it takes one half life for 50% completion and two half lives for 75% completion. Clearly, the reaction is first order w.r.t. P.
Hence, overall order = 0 +1 = 1 (D).